Regulatory Affairs Services
The activity of Help S.A. in a variety of geographical territories has facilitated the development of a competitive Regulatory Affairs (RA) department, with extensive experience in the registration of pharmaceuticals, medical devices, food supplements and cosmetics locally & internationally.
Our Regulatory Department is currently managing more than 150 MAs in Greece and more than 400 MAs in foreign countries.
We can offer you our consulting support or full regulatory affairs services in:
- Development, update and conversion of drug product dossiers in electronic CTD format or in a specific format as per the selected country requirements
- Registration of drug products, medical devices, cosmetics & food supplements to Health Authorities
- Post-authorization activities for the management of the product licenses, including renewals, variations and notifications.
QC Services
Quality Control Testing Release
We can undertake the Quality Control testing and Release of your product. We work closely with your team to transfer the analytical methods and provide guidance on requirements for release to EU markets.
Analytical Validations/Method Development
Help is committed to quality and scientific excellence. We work with you to transfer your methods, develop more reliable methods, perform stress tests on Finished Products and APIs. A wide range of modernized analytical equipment is available in our laboratory, qualified and validated to ensure testing meets cGMP standards.
Stability Programs/Photostability Studies
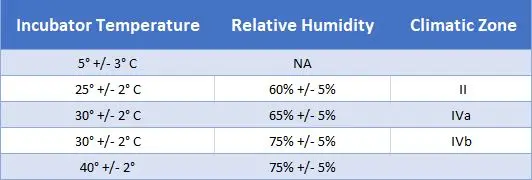
We design and monitor your stability studies, while keeping the integrity of your data at the highest level. Our experts in cooperation with our analytical team will develop stability protocols that are specific to the needs of your study. We will design these with set pull points, incubation temperatures, and testing requirements for each pull point. A final report will be provided at the end of each study, but you can also receive interim reports throughout the stability study after review and approval from our quality department. The validated chambers that are available to clients for storage are listed below, but if you need an additional temperature point that we do not currently have, please contact us and share your needs and requirements. We work closely with you and our quality department will ensure that your study is designed to give you the most effective measures of your drug’s stability (including in-use stability, freeze thaw studies).
Photostability Studies
Help can design a photostability study for your product according to ICH Q1B, find the suitable container for your product or set the proper limitations on storage conditions.
Efficacy Testing on Antimicrobial Agents
Our microbiological laboratory can evaluate the efficacy of antimicrobial agents to a pharmaceutical preparation, our experts can then propose the most suitable quantity of an antimicrobial agent. The study will evaluate the efficacy on stability samples up to shelf life and at several climatic zones.
EU Batch Release
As an EU based site, we can provide EU Batch Release for your product in compliance with laws in force in the EU, in accordance with the requirements of the marketing authorization (MA) and with Good Manufacturing Practice (GMP).
- QP-certified batch release analysis
- Batch release for medicinal products in compliance with Good Manufacturing Practice (GMP) requirements and for medical devices
- Release of medicinal products for clinical trials (investigational medicinal products)
- Import release for medicinal products from companies outside of the EU / EEA
